The VOICE study tested whether different ways of working with Veterans and their primary care providers helped to improve pain.
Goals of the VOICE study
The study’s overarching goals were to improve both the effectiveness and safety of pain management for Veterans living with moderate to severe chronic pain. The study compared two active interventions (two clinical pain management team models).
VOICE is no longer open for enrollment
The study completed enrollment in 2021, and the final VOICE Veterans completed their 12-month participation in Spring 2022. The study team is currently analyzing data collected during the active study intervention.
Who was eligible to enroll in VOICE? What did enrollees do during their 12-month participation?
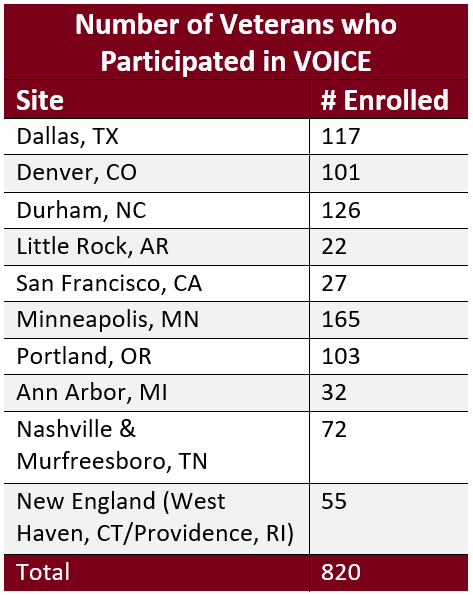
The study enrolled 820 Veterans receiving care at 10 selected VA sites who met the following criteria:
- Severe pain despite treatment with opioid medications
- Willingness to be assigned by chance to either of the study’s two pain care groups
- Availability to participate for one year
After enrolling, each Veteran was randomly assigned to work with one of two VA pain care teams for 12 months - the Integrated Pain Care Team or the Pharmacist Pain Care team.
Enrolled veterans were also asked to complete research interviews by telephone every 3 months for 12 months. Research interview questions focused on measuring:
- Pain and quality of life
- Sleep, fatigue, and mood
- Medication benefits or side effects
- Satisfaction with pain management
The Integrated Pain Care Team & Pharmacist Pain Care teams
Each was comprised each team of VA clinicians. The Integrated Pain Care Team (IPT) included a medical provider, mental health clinician, and a clinical pharmacist or rehabilitation therapist. The IPT intervention was defined by interdisciplinary team care planning, an emphasis on nondrug therapies, & behavioral activation sessions (coaching calls). The Pharmacist Pain Care Team included a clinical pharmacist care manager and consulting physician and was defined by structured symptom monitoring and pain medication optimization.
Both teams collaborated with primary care teams; provided patient-centered informational materials; practiced shared decision-making about pain therapies and opioid changes; and provided individualized opioid tapering recommendations to Veterans.
Study results are pending
We are currently analyzing data and will continue with analysis into 2023. The VOICE study is registered at www.clinicaltrials.gov; study number NCT03026790. Main study results will be posted to www.clinicaltrials.gov. You may also follow this website and the VOICE publications tab for published results.
VOICE study funder
The VOICE study was funded by the Patient-Centered Outcomes Research Institute (PCORI), the leading funder of patient-centered comparative clinical effectiveness research in the United States. PCORI is an independent, non-profit research organization that “seeks to empower patients and others with actionable information about their health and healthcare choices." Visit https://www.pcori.org/ for more information or to subscribe for email alerts to follow their studies.
The VOICE study is approved by the VA’s Central Institutional Review Board (IRB). VA Central Institutional Review Board (IRB)